Wet etching is a cornerstone of microfabrication, enabling the precise shaping of materials at the microscale. In the context of stainless steel, a material prized for its corrosion resistance, mechanical strength, and versatility, wet etching presents unique opportunities and challenges. This article explores the process stability of wet etching for creating stainless steel microstructures, delving into the chemical, physical, and engineering principles that govern the process. It examines the factors influencing stability, compares recent advancements, and provides detailed tables to contextualize performance metrics across various etching conditions. The discussion is grounded in scientific rigor, drawing from established research to offer a comprehensive understanding suitable for researchers, engineers, and practitioners in materials science and microengineering.
Introduction to Wet Etching and Stainless Steel Microstructures
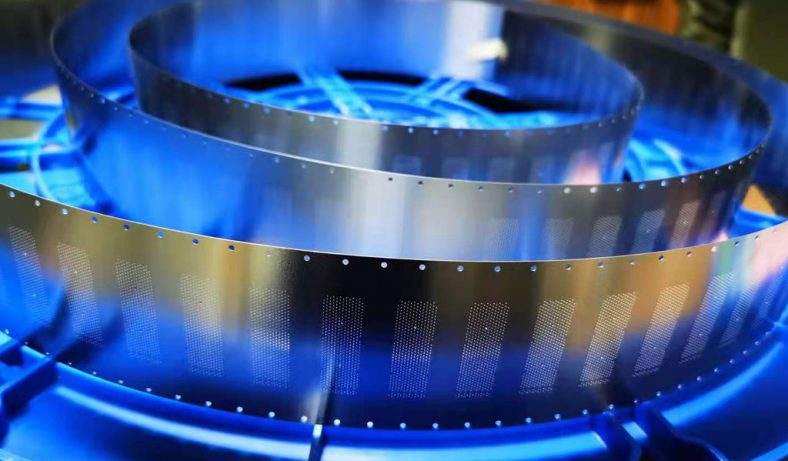
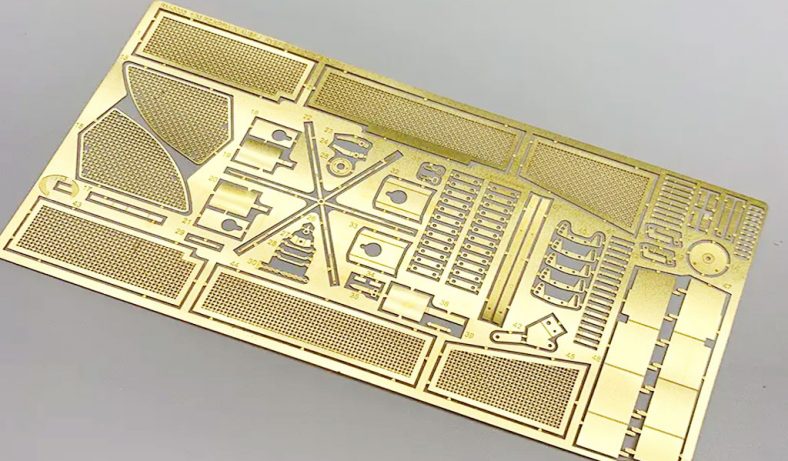
Wet etching is a subtractive manufacturing process that uses liquid chemical etchants to selectively remove material from a substrate, creating intricate patterns or structures. Unlike dry etching, which relies on plasma or gaseous etchants, wet etching employs aqueous solutions, offering simplicity, cost-effectiveness, and high selectivity for certain materials. In microfabrication, wet etching is critical for producing components such as microfluidic channels, sensors, and precision mechanical parts.
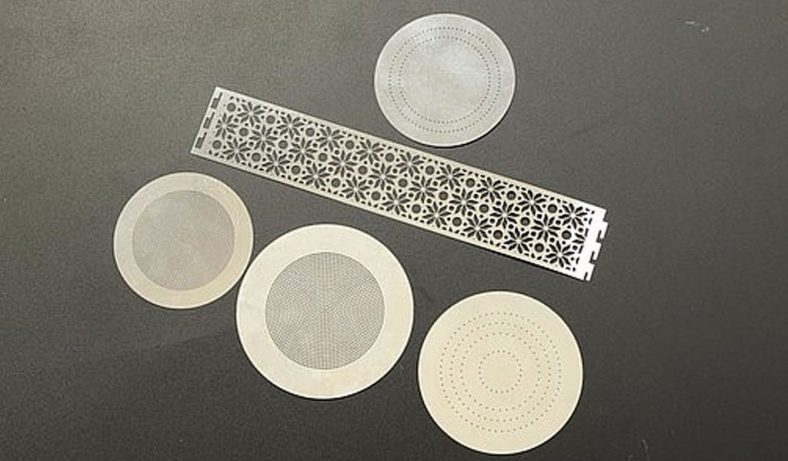
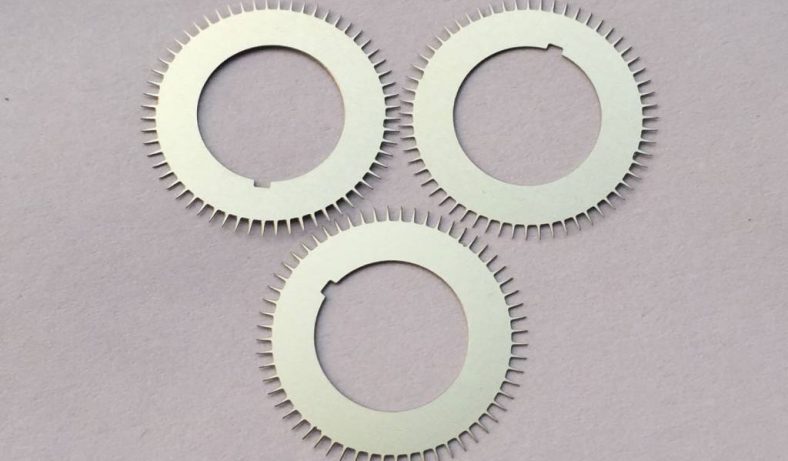
Stainless steel, an iron-based alloy containing at least 10.5% chromium, is widely used in industries ranging from aerospace to biomedical engineering due to its durability, corrosion resistance, and aesthetic appeal. Common grades, such as 304 (austenitic) and 420 (martensitic), are selected based on their microstructure and mechanical properties. Microstructures in stainless steel—features with dimensions on the order of micrometers—are increasingly demanded for applications like microelectromechanical systems (MEMS), medical implants, and microreactors. Achieving stable wet etching processes for these microstructures is essential to ensure reproducibility, precision, and quality.
Process stability in wet etching refers to the consistency of etching outcomes—such as etch rate, surface roughness, and dimensional accuracy—under varying conditions. Stability is influenced by etchant chemistry, temperature, material properties, and process parameters. For stainless steel, the presence of a passive chromium oxide layer and the alloy’s complex microstructure complicate etching, necessitating careful control to maintain stability. This article synthesizes recent findings to elucidate the mechanisms and challenges of stable wet etching, providing a foundation for optimizing microfabrication processes.
Fundamentals of Wet Etching Stainless Steel
Chemical Principles of Wet Etching
Wet etching of stainless steel involves the chemical dissolution of metal atoms from the surface, driven by reactions between the etchant and the alloy’s constituents. The primary etchant for stainless steel is ferric chloride (FeCl₃), which oxidizes iron and other metals, forming soluble complexes that are removed from the surface. The reaction can be represented as:
[ \text{Fe} + 2\text{FeCl}_3 \rightarrow 3\text{FeCl}_2 ]
Chromium and nickel, key components of stainless steel, also react, though at different rates due to their chemical stability. Chromium’s passive oxide layer (Cr₂O₃) resists etching, requiring etchants to penetrate or disrupt this layer. Nitric acid (HNO₃) or hydrochloric acid (HCl) is often added to ferric chloride to enhance etching by breaking down the oxide layer or increasing the solution’s aggressiveness.
Etch selectivity—the ability to etch one material faster than another—is critical for microstructural control. In stainless steel, selectivity is influenced by the alloy’s phase (e.g., austenite, ferrite, or martensite) and the etchant’s composition. For example, austenitic stainless steels (e.g., 304) etch more uniformly with ferric chloride than martensitic grades (e.g., 420), which may exhibit phase-specific etching due to their tempered microstructure.
Physical Mechanisms
The etching process is governed by mass transport and surface kinetics. Etchant ions must diffuse to the metal surface, react, and remove reaction products. This process is affected by:
- Diffusion: The rate at which etchant ions reach the surface depends on concentration gradients and solution agitation. Stagnant solutions lead to uneven etching, while excessive agitation may cause turbulence, affecting uniformity.
- Surface Reactions: The reaction rate is temperature-dependent, following Arrhenius behavior, where higher temperatures accelerate etching but may reduce selectivity.
- Mask Adhesion: Photolithography is used to apply a photoresist mask, defining the etched pattern. Poor mask adhesion can lead to undercutting, where etchant seeps beneath the mask, compromising dimensional accuracy.
Microstructural Considerations
Stainless steel’s microstructure—comprising grains, phases, and inclusions—significantly impacts etching stability. Austenitic stainless steels have a face-centered cubic (FCC) structure, promoting uniform etching, while ferritic (body-centered cubic, BCC) and martensitic (body-centered tetragonal, BCT) grades may exhibit anisotropic etching due to grain orientation or phase boundaries. Sensitization, where chromium carbides precipitate at grain boundaries, can increase susceptibility to intergranular attack, affecting etch uniformity.
Factors Influencing Process Stability
Etchant Composition and Concentration
The choice of etchant and its concentration profoundly affects etching stability. Ferric chloride is the most common etchant, with concentrations typically ranging from 32°Bé to 44°Bé (Baumé scale, indicating density). Higher concentrations increase etch rate but may reduce selectivity, leading to over-etching or rough surfaces. Additives like HCl or HNO₃ modify the etchant’s reactivity:
- HCl Addition: Enhances oxide layer removal, increasing etch depth but potentially causing pitting if not controlled.
- HNO₃ Addition: Stabilizes the etch rate by passivating certain phases, improving surface smoothness but slowing the process.
A study by Çakır et al. (2025) investigated four ferric chloride concentrations (32°Bé, 36°Bé, 40°Bé, 44°Bé) with and without HCl, finding that 40°Bé with 150 mL/L HCl at 40°C optimized etch depth and surface roughness for 304 stainless steel.
Etching Temperature
Temperature influences reaction kinetics and diffusion rates. Higher temperatures accelerate etching but may destabilize the process by promoting side reactions or mask degradation. For example, at 50°C, ferric chloride etches 304 stainless steel 30% faster than at 30°C, but surface roughness increases due to aggressive bubble formation. Optimal temperatures typically range from 35°C to 45°C, balancing speed and control.
Immersion Time
Etching time determines the depth and quality of the microstructure. Short times may result in incomplete etching, while prolonged exposure causes over-etching, leading to dimensional inaccuracies. For instance, a 30-minute etch with 350 g/L FeCl₃ and 100 mL/L HNO₃ on 304 stainless steel achieves a depth of 160 µm with minimal roughness, while 180 minutes increases roughness by 20%.
Material Properties
The alloy’s composition and microstructure dictate etching behavior. Austenitic grades (e.g., 304, 316) resist pitting better than ferritic (e.g., 430) or martensitic (e.g., 420) grades due to their homogeneous FCC structure. Cold-worked stainless steel etches faster than annealed samples because of increased defect density, which enhances etchant penetration.
Process Parameters
- Agitation: Controlled stirring or ultrasonic agitation ensures uniform etchant distribution, reducing concentration gradients that cause uneven etching.
- Mask Quality: High-resolution photomasks (e.g., UV-resistant polymer films) prevent undercutting, ensuring precise microstructures.
- Equipment Design: Modern etching machines, such as those with spray nozzles, deliver consistent etchant flow, improving stability over dip-tank methods.
Challenges in Achieving Process Stability
Undercutting and Dimensional Control
Undercutting occurs when etchant removes material beneath the mask, widening features beyond the intended design. This is a significant challenge in wet etching, as isotropic etching removes material equally in all directions. For stainless steel, undercutting is exacerbated by the alloy’s resistance to etching, requiring prolonged exposure that increases lateral etching. Advanced masking techniques, such as multi-layer photoresists, mitigate undercutting but add complexity.
Surface Roughness
Surface roughness affects the functionality of microstructures, particularly in applications like microfluidics, where smooth surfaces reduce flow resistance. Ferric chloride etching often produces rough surfaces due to uneven dissolution of grains or phases. Additives like HNO₃ or post-etch polishing can improve smoothness, but they increase process time and cost.
Pitting and Corrosion
Pitting corrosion, where localized cavities form, is a risk in stainless steel etching, especially in chloride-based etchants. Pitting is influenced by etchant aggressivity, alloy composition, and surface defects. For example, 316L stainless steel, with higher molybdenum content, resists pitting better than 304 in HCl-containing etchants.
Environmental and Safety Concerns
Wet etching generates hazardous waste, including heavy metal ions and acidic solutions, requiring careful disposal. Ferric chloride is recyclable, but additives like HCl increase waste treatment complexity. Safety protocols, such as fume hoods and protective equipment, are essential to handle corrosive etchants.
Recent Advances in Wet Etching Stability
Optimized Etchant Formulations
Recent studies have explored novel etchant formulations to enhance stability. For instance, combining FeCl₃ with HNO₃ and HCl in precise ratios (e.g., 350 g/L FeCl₃, 150 mL/L HCl, 100 mL/L HNO₃) achieves a balance of etch rate and surface quality for 304 stainless steel. Additionally, glycerol-ethanolic solutions with FeCl₂ and AlCl₃ have been tested for pitting corrosion studies, offering controlled etching for defect analysis.
Electrochemical Wet Etching
Electrochemical wet etching, where an external voltage drives the etching process, improves stability by controlling reaction rates. A 2025 study demonstrated that 3% oxalic acid with 3V DC produced high-aspect-ratio micro-holes in 304 stainless steel with an aspect ratio of 0.82, compared to 0.3 for conventional wet etching. This method reduces undercutting and enhances anisotropy, making it suitable for complex microstructures.
Laser-Assisted Etching
Electrochemical etching using laser masking (EELM) eliminates the need for photolithography by creating temporary oxide masks via laser marking. This technique, applied to stainless steel, achieves multilayered structures with high precision, as the laser-marked areas resist anodic dissolution. EELM is particularly promising for rapid prototyping, though it requires optimization to minimize material ablation.
Photochemical Etching
Photochemical etching, using UV-exposed photoresists, has advanced with digital tooling, allowing rapid design iterations. Companies like Precision Micro report etching 304 stainless steel to tolerances below ±10% of material thickness, producing burr-free meshes for radiation detectors. This method enhances stability by eliminating mechanical stress and heat, preserving the alloy’s properties.
Comparative Analysis of Etching Conditions
To illustrate the impact of various parameters on process stability, the following tables compare recent experimental results for wet etching of stainless steel microstructures. These tables focus on etch rate, surface roughness, and dimensional accuracy across different etchants, temperatures, and alloy grades.
Table 1: Etch Rate Comparison for 304 Stainless Steel
| Etchant Composition | Concentration | Temperature (°C) | Etch Rate (µm/min) | Reference |
|---|---|---|---|---|
| FeCl₃ | 32°Bé | 40 | 2.5 | |
| FeCl₃ | 40°Bé | 40 | 3.2 | |
| FeCl₃ + HCl | 40°Bé + 150 mL/L | 40 | 4.0 | |
| FeCl₃ + HNO₃ | 350 g/L + 100 mL/L | 40 | 3.8 | |
| Oxalic Acid (Electrochemical) | 3% | 25 | 1.8 |
Notes: Etch rate increases with FeCl₃ concentration and HCl addition, but electrochemical etching is slower due to controlled dissolution. Higher temperatures generally enhance etch rate but require careful monitoring to avoid roughness.
Table 2: Surface Roughness Comparison
| Alloy Grade | Etchant | Temperature (°C) | Roughness (Ra, µm) | Reference |
|---|---|---|---|---|
| 304 | FeCl₃ (40°Bé) | 40 | 0.45 | |
| 304 | FeCl₃ + HCl | 40 | 0.60 | |
| 316L | H₂SO₄ (4M) | 25 | 0.80 | |
| 304 | FeCl₃ + HNO₃ | 40 | 0.38 | |
| 420 | HNO₃ (2M) | 30 | 0.55 |
Notes: HNO₃ addition reduces roughness compared to HCl, while 316L exhibits higher roughness in H₂SO₄ due to phase-specific etching. Austenitic grades generally achieve smoother surfaces than martensitic grades.
Table 3: Dimensional Accuracy (Undercutting)
| Etching Method | Mask Type | Alloy | Undercutting (µm) | Reference |
|---|---|---|---|---|
| Chemical (FeCl₃) | Photoresist | 304 | 15 | |
| Photochemical | UV Polymer | 304 | 8 | |
| Electrochemical (Oxalic Acid) | Resist | 304 | 5 | |
| EELM | Laser Oxide | 316L | 3 | |
| Chemical (HNO₃) | Photoresist | 420 | 20 |
Notes: Electrochemical and laser-assisted methods significantly reduce undercutting, enhancing dimensional accuracy. Photochemical etching outperforms traditional chemical etching due to digital tooling precision.
Applications of Stable Wet Etching
Microelectromechanical Systems (MEMS)
Stable wet etching enables the fabrication of stainless steel MEMS components, such as microgears and actuators, with high precision. The corrosion resistance of 316L makes it ideal for harsh environments, while photochemical etching ensures stress-free structures.
Biomedical Devices
Microstructured stainless steel is used in surgical instruments, stents, and microfluidic diagnostic devices. Stable etching ensures smooth surfaces that minimize tissue irritation and precise channels for fluid control. For example, 304 stainless steel meshes for filtration are etched with tolerances below 10 µm.
Aerospace and Automotive
In aerospace, wet-etched stainless steel components, such as heat exchangers and optical encoders, benefit from high strength and corrosion resistance. The ability to produce complex geometries without thermal stress is critical for these applications.
Future Directions
Sustainable Etching Processes
Reducing the environmental impact of wet etching is a priority. Research into recyclable etchants and waste-neutralizing additives is ongoing. For instance, ferric chloride can be regenerated through oxidation, minimizing waste.
Integration with Additive Manufacturing
Combining wet etching with additive manufacturing (e.g., 3D-printed stainless steel) could enable hybrid microfabrication, where etching refines printed structures. This approach is underexplored but holds potential for complex, multi-material devices.
Machine Learning Optimization
Machine learning models are being developed to predict etch outcomes based on parameters like etchant concentration and temperature. These models could enhance stability by optimizing conditions in real-time, reducing trial-and-error in process development.
Conclusion
The process stability of wet etching in stainless steel microstructures is a multifaceted challenge, requiring precise control of chemical, physical, and material factors. Advances in etchant formulations, electrochemical techniques, and laser-assisted methods have significantly improved stability, enabling high-precision microstructures for diverse applications. Comparative analyses reveal that optimized conditions—such as 40°Bé FeCl₃ with HNO₃ at 40°C—balance etch rate, surface quality, and dimensional accuracy. Future research should focus on sustainable practices and integration with emerging technologies to further enhance the reliability and versatility of wet etching in microfabrication.