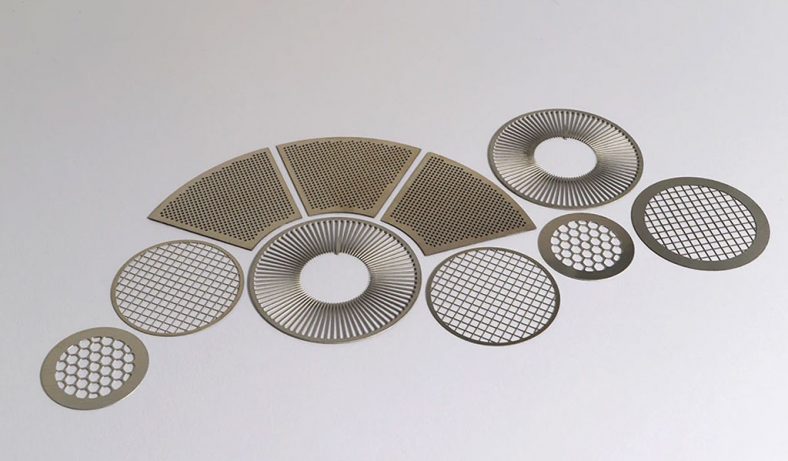
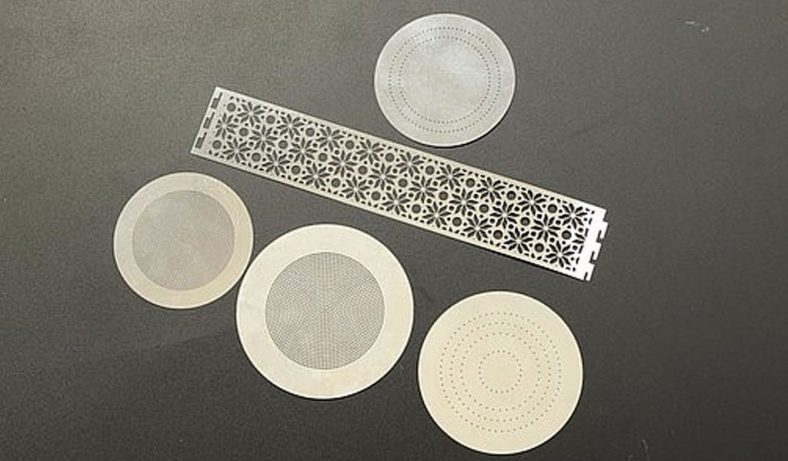
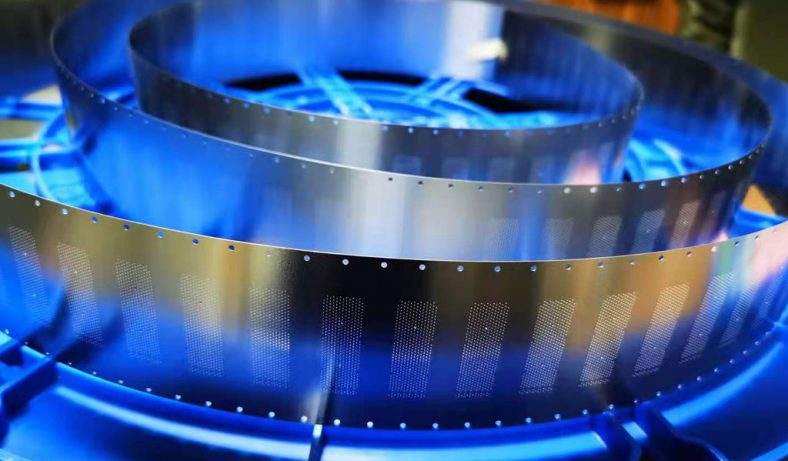
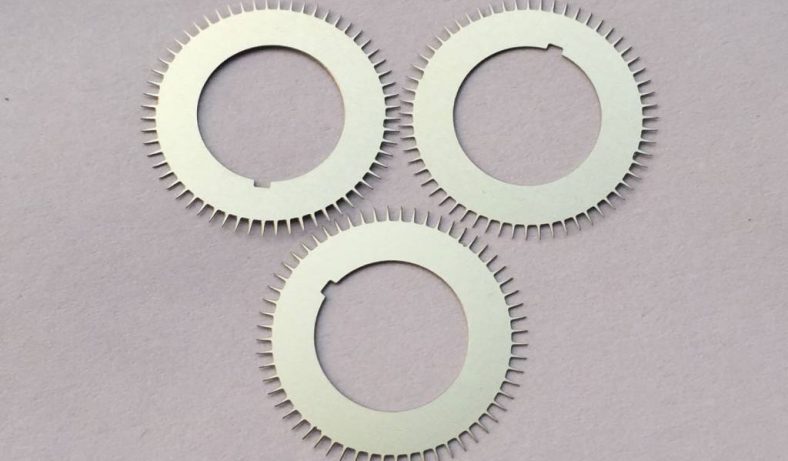
Photochemical etching (PCE), also known as photochemical machining or chemical etching, is a subtractive manufacturing process used to fabricate precise metal components by selectively removing material using chemical etchants and photoresist masks. This technique is particularly valuable for producing intricate micro-hole patterns in thin metal sheets, offering advantages over traditional methods like stamping, laser cutting, or mechanical drilling. In the context of zinc alloys—materials valued for their corrosion resistance, castability, and cost-effectiveness—photochemical etching enables the creation of micro-holes with high precision, minimal mechanical stress, and burr-free edges. The process is widely applied in industries such as electronics, aerospace, medical devices, and microfluidics, where micro-hole arrays are critical for components like filters, meshes, and fluidic channels.
The success of photochemical etching for zinc alloy micro-hole processing hinges on the precise control of process parameters, which directly influence the quality, accuracy, and efficiency of the resulting components. These parameters include etchant composition, etching temperature, etching time, photoresist properties, and UV exposure conditions, among others. This article provides a comprehensive exploration of parameter control in photochemical etching of zinc alloys for micro-hole processing, detailing the underlying principles, parameter effects, optimization strategies, and practical applications. Through detailed explanations, comparative tables, and references to recent studies, the article aims to serve as a definitive resource for researchers, engineers, and practitioners in the field.
Fundamentals of Photochemical Etching
Photochemical etching is a non-contact, chemical-based process that combines photolithography and wet etching to produce precise metal parts. The process begins with a metal sheet, in this case, a zinc alloy, which is cleaned and coated with a photosensitive resist. A photographic mask, or photo-tool, containing the desired micro-hole pattern is placed over the resist-coated metal. Ultraviolet (UV) light is then used to expose the resist through the mask, hardening the exposed areas (in negative-tone resists) or softening them (in positive-tone resists). The unexposed resist is developed and removed, exposing the underlying metal. The sheet is then immersed in or sprayed with an etchant, typically an acidic solution, which selectively dissolves the unprotected metal to form micro-holes. Finally, the remaining resist is stripped, and the part is cleaned and inspected.
Zinc alloys, composed primarily of zinc with additives like aluminum, copper, or magnesium (e.g., Zamak alloys), are well-suited for photochemical etching due to their chemical reactivity and uniform etching behavior. The process is particularly effective for thin sheets (0.013–2.032 mm), allowing for high-resolution micro-hole patterns with diameters as small as 0.1 mm. Unlike mechanical methods, photochemical etching avoids thermal or mechanical stress, preserving the material’s properties and enabling complex geometries without burrs or tool wear.
The etching process is governed by electrochemical reactions between the etchant and the zinc alloy surface. For zinc alloys, common etchants include ferric chloride (FeCl₃), nitric acid (HNO₃), or hydrochloric acid (HCl), which oxidize and dissolve the metal. The reaction for zinc in ferric chloride, for example, can be simplified as:
[ \text{Zn} + 2\text{FeCl}_3 \rightarrow \text{ZnCl}_2 + 2\text{FeCl}_2 ]
This reaction highlights the importance of etchant concentration and composition, as they determine the rate of material removal and the quality of the etched features. Other parameters, such as temperature and exposure time, further modulate the etching dynamics, making parameter control a critical aspect of achieving desired micro-hole characteristics.
Key Parameters in Photochemical Etching of Zinc Alloys
The photochemical etching process involves multiple interdependent parameters that must be carefully controlled to achieve optimal micro-hole quality. These parameters can be broadly categorized into chemical, physical, and process-related factors. Below, each key parameter is discussed in detail, with an emphasis on its role in zinc alloy micro-hole processing.
Etchant Composition and Concentration
The choice of etchant and its concentration is the cornerstone of photochemical etching, as it directly affects the etching rate, surface finish, and dimensional accuracy of micro-holes. For zinc alloys, ferric chloride is the most commonly used etchant due to its effectiveness, cost-efficiency, and compatibility with a wide range of metals. Other etchants, such as nitric acid or hydrochloric acid, may be used for specific applications requiring faster etching or smoother surfaces.
- Ferric Chloride (FeCl₃): Typically used at concentrations of 300–700 g/L, ferric chloride provides a balance between etching speed and control. Higher concentrations increase the etching rate but may lead to undercutting (lateral etching beneath the resist), reducing hole precision. Lower concentrations slow the process but enhance uniformity.
- Nitric Acid (HNO₃): Used at 5–20% concentration, nitric acid is effective for rapid etching but can produce rougher surfaces due to its aggressive reaction with zinc. It is often combined with other acids to moderate its activity.
- Hydrochloric Acid (HCl): At 10–30% concentration, HCl is used for fine feature etching, offering high resolution but requiring careful control to avoid excessive gas evolution, which can disrupt the resist.
The etchant’s pH and redox potential also influence its performance. For instance, a study by Mallik et al. (2023) found that an etchant concentration of 700 g/L at 60°C optimized the material removal rate (MRR) for zinc-based composites, achieving an etch factor (ratio of vertical to lateral etching) of 1.8. Table 1 compares common etchants for zinc alloy etching, highlighting their properties and typical applications.
Table 1: Comparison of Etchants for Zinc Alloy Photochemical Etching
| Etchant | Concentration Range | Etching Rate (µm/min) | Surface Finish | Advantages | Limitations | Applications |
|---|---|---|---|---|---|---|
| Ferric Chloride | 300–700 g/L | 0.5–1.0 | Smooth | Cost-effective, widely compatible | Risk of undercutting at high conc. | Micro-holes, meshes, filters |
| Nitric Acid | 5–20% | 1.0–2.0 | Moderate | Fast etching, high throughput | Rough surfaces, gas evolution | Rapid prototyping, coarse features |
| Hydrochloric Acid | 10–30% | 0.8–1.5 | Very Smooth | High resolution, fine features | Requires precise control, corrosive | Microfluidics, precision filters |
| Mixed Acids (HNO₃ + HCl) | 5–15% each | 1.2–2.5 | Smooth | Balanced speed and quality | Complex preparation, safety concerns | High-precision components |
Etching Temperature
Temperature plays a critical role in controlling the etching rate and the quality of micro-holes. Higher temperatures accelerate the chemical reaction between the etchant and zinc alloy, increasing the material removal rate but potentially compromising precision due to enhanced lateral etching. Conversely, lower temperatures slow the process, improving control over feature dimensions but extending processing time.
For zinc alloys, etching is typically performed at 20–60°C. A temperature of 40–50°C is often optimal, balancing speed and accuracy. For example, a study on photochemical machining of aluminum/zinc composites found that 60°C maximized the etch factor but increased surface roughness by 15% compared to 40°C. Temperature also affects etchant viscosity and diffusion, influencing the uniformity of micro-hole arrays across large sheets.
Table 2: Effect of Etching Temperature on Zinc Alloy Micro-Hole Processing
| Temperature (°C) | Etching Rate (µm/min) | Surface Roughness (Ra, µm) | Etch Factor | Processing Time (min/cm²) | Notes |
|---|---|---|---|---|---|
| 20 | 0.3–0.5 | 0.2–0.3 | 2.0–2.5 | 10–15 | High precision, slow process |
| 40 | 0.6–0.9 | 0.3–0.4 | 1.8–2.2 | 5–8 | Optimal for most applications |
| 60 | 1.0–1.5 | 0.4–0.6 | 1.5–1.8 | 3–5 | Fast, risk of undercutting |
Etching Time
Etching time determines the depth and diameter of micro-holes, as prolonged exposure to the etchant increases material removal. For zinc alloys, etching times typically range from 3 to 15 minutes, depending on the sheet thickness (0.013–2.032 mm) and desired hole depth. Over-etching can lead to enlarged holes or loss of dimensional tolerance, while under-etching results in incomplete hole formation.
The relationship between etching time and hole diameter is non-linear due to isotropic etching behavior, where lateral etching occurs alongside vertical etching. A study by Precision Micro (2021) noted that for 0.25 mm thick zinc alloy sheets, an etching time of 12 minutes at 40°C with 500 g/L ferric chloride achieved a minimum feature size of 0.1 mm with ±0.025 mm tolerance. Monitoring and controlling etching time is thus critical for high-density micro-hole arrays.
Photoresist Properties
The photoresist serves as a protective mask, defining the micro-hole pattern during etching. Its adhesion, resolution, and chemical resistance are pivotal for achieving precise features. Common photoresists for zinc alloy etching include dry film resists (e.g., DuPont Riston) and liquid resists, each with distinct properties:
- Dry Film Resists: Applied as a laminated sheet, these resists offer uniform thickness (25–50 µm) and high durability, making them suitable for large-scale production. They are less prone to pinhole defects but may limit resolution for sub-0.1 mm features.
- Liquid Resists: Spin-coated or sprayed, liquid resists provide higher resolution (down to 0.05 mm) but require precise application to avoid thickness variations. They are ideal for prototyping or ultra-fine micro-holes.
The resist’s exposure to UV light must be optimized to ensure complete polymerization (for negative resists) or degradation (for positive resists). Underexposure can lead to resist failure during etching, while overexposure may reduce resolution. A typical UV exposure dose for zinc alloy etching is 100–300 mJ/cm², depending on the resist type and thickness.
UV Exposure Conditions
UV exposure transfers the micro-hole pattern from the photo-tool to the resist. Key parameters include light intensity, wavelength, and exposure time. Most photochemical etching systems use UV light at 365–405 nm, with intensities of 10–50 mW/cm². The photo-tool, typically a glass or polyester film with a high-contrast pattern, must align precisely with the metal sheet to avoid misalignment errors.
Collimated UV light sources improve pattern fidelity by reducing light scattering, which is critical for micro-holes with diameters below 0.2 mm. Misalignment or uneven exposure can result in distorted or incomplete holes. For zinc alloys, a study by MET Manufacturing Group (2023) emphasized the importance of maintaining a 1:1 aspect ratio between the photo-tool and resist to achieve tolerances of ±0.01 mm for 0.15 mm diameter holes.
Sheet Thickness and Alloy Composition
The thickness of the zinc alloy sheet and its composition influence etching behavior. Thinner sheets (0.013–0.5 mm) etch faster and support finer features, while thicker sheets (up to 2.032 mm) require longer etching times and may exhibit reduced resolution due to increased undercutting. Common zinc alloys for micro-hole processing include:
- Zamak 3 (Zn-4%Al): Widely used for its strength and castability, Zamak 3 etches uniformly with ferric chloride, achieving hole diameters as small as 0.12 mm.
- Zamak 5 (Zn-4%Al-1%Cu): Offers higher hardness but slightly slower etching rates due to copper’s lower reactivity.
- ZA-8 (Zn-8%Al): Higher aluminum content improves corrosion resistance but may increase surface roughness during etching.
The alloy’s microstructure, including grain size and phase distribution, affects etching isotropy. Fine-grained alloys etch more uniformly, while coarse-grained structures may lead to uneven hole edges.
Table 3: Influence of Zinc Alloy Composition on Micro-Hole Etching
| Alloy | Composition | Etching Rate (µm/min) | Minimum Hole Diameter (mm) | Surface Roughness (Ra, µm) | Applications |
|---|---|---|---|---|---|
| Zamak 3 | Zn-4%Al | 0.6–0.9 | 0.12 | 0.3–0.4 | Filters, electronic connectors |
| Zamak 5 | Zn-4%Al-1%Cu | 0.5–0.8 | 0.15 | 0.4–0.5 | Automotive sensors, meshes |
| ZA-8 | Zn-8%Al | 0.4–0.7 | 0.18 | 0.5–0.6 | Corrosion-resistant microfluidic channels |
Optimization Strategies for Parameter Control
Achieving high-quality micro-holes in zinc alloys requires systematic optimization of etching parameters. This section explores strategies for balancing etching rate, dimensional accuracy, and surface quality, drawing on experimental findings and industry practices.
Design of Experiments (DoE)
The Design of Experiments (DoE) approach is widely used to optimize photochemical etching parameters. By systematically varying factors like etchant concentration, temperature, and time, DoE identifies interactions and optimal settings. A study by Mallik et al. (2023) applied Gray Relational Analysis (GRA) with DoE to optimize zinc alloy etching, finding that a combination of 700 g/L ferric chloride, 60°C, and 12 minutes maximized MRR and etch factor while minimizing undercut.
A typical DoE setup for zinc alloy micro-hole etching might include:
- Factors: Etchant concentration (300, 500, 700 g/L), temperature (20, 40, 60°C), time (5, 10, 15 min).
- Responses: Etching rate, hole diameter tolerance, surface roughness, etch factor.
- Design: Full factorial or Taguchi method to reduce experimental runs.
Etchant Recycling and Regeneration
Etchant degradation over time can lead to inconsistent etching results. Recycling and regeneration systems maintain etchant potency by removing reaction byproducts (e.g., ZnCl₂) and replenishing active species (e.g., Fe³⁺). For ferric chloride, regeneration involves adding chlorine gas or hydrogen peroxide to oxidize Fe²⁺ back to Fe³⁺. This ensures consistent etching rates and reduces waste, enhancing process sustainability.
Real-Time Monitoring and Feedback
Advanced etching systems incorporate real-time monitoring of parameters like etchant pH, temperature, and metal ion concentration. Sensors and automated feedback loops adjust conditions to maintain optimal performance. For example, Precision Micro’s etching lines use multi-chambered machines with spray nozzles and conveyors, monitored to achieve ±0.025 mm tolerances for zinc alloy micro-holes.
Photo-Tool Design and Alignment
The photo-tool’s resolution and alignment are critical for micro-hole precision. High-contrast, laser-plotted polyester films are preferred for cost-effectiveness, while glass photo-tools offer superior durability for high-volume production. Automated alignment systems with sub-micron precision minimize registration errors, ensuring uniform hole patterns across large sheets.
Challenges in Micro-Hole Processing
Despite its advantages, photochemical etching of zinc alloys for micro-hole processing faces several challenges:
- Undercutting: Lateral etching beneath the resist can enlarge hole diameters, reducing precision. This is mitigated by optimizing etchant concentration and using high-adhesion resists.
- Surface Roughness: Aggressive etchants or high temperatures may increase roughness, affecting fluidic or optical performance. Smoother surfaces are achieved with lower temperatures and milder etchants like HCl.
- Resist Failure: Pinholes or resist delamination can lead to unwanted etching. This is addressed by improving resist application techniques and ensuring uniform UV exposure.
- Material Limitations: Zinc alloys with high aluminum or copper content may etch unevenly due to phase segregation. Pre-etching surface treatments, such as electropolishing, can enhance uniformity.
Applications of Zinc Alloy Micro-Hole Components
Photochemically etched zinc alloy micro-holes find applications across diverse industries, leveraging their precision, durability, and cost-effectiveness:
- Electronics: Micro-hole arrays in zinc alloy sheets serve as EMI/RFI shielding, connectors, and lead frames. Their high conductivity and corrosion resistance make them ideal for compact devices.
- Microfluidics: Micro-holes form channels and filters in lab-on-a-chip devices, enabling precise fluid manipulation for medical diagnostics and chemical analysis.
- Aerospace: Lightweight zinc alloy meshes with micro-holes are used in filtration systems and sensor arrays, benefiting from the process’s ability to produce complex geometries.
- Automotive: Micro-hole components in fuel cell plates and sensors enhance efficiency and performance, leveraging zinc’s recyclability and etching’s scalability.
Recent Advances and Future Directions
Recent studies have advanced photochemical etching techniques for zinc alloys, focusing on sustainability, precision, and scalability. Innovations include:
- Environmentally Friendly Etchants: Research into biodegradable or less toxic etchants, such as citric acid-based solutions, aims to reduce environmental impact.
- Hybrid Processes: Combining photochemical etching with laser micromachining or plasma etching enhances resolution for sub-0.1 mm holes.
- Machine Learning Optimization: Artificial neural networks (ANNs) and GRA are increasingly used to predict and optimize etching outcomes, as demonstrated in studies on zinc-based composites.
Future directions include integrating photochemical etching with additive manufacturing for hybrid micro-hole components, developing in-situ etching control systems, and expanding applications in flexible electronics and wearable devices.
Conclusion
Photochemical etching is a versatile and precise method for fabricating micro-hole arrays in zinc alloys, offering unmatched advantages in resolution, cost, and scalability. By carefully controlling parameters such as etchant composition, temperature, time, photoresist properties, and UV exposure, manufacturers can achieve high-quality components with tolerances as tight as ±0.01 mm. Optimization strategies like DoE, etchant regeneration, and real-time monitoring further enhance process reliability. Despite challenges like undercutting and resist failure, ongoing innovations in etchants, hybrid processes, and AI-driven optimization promise to expand the capabilities of photochemical etching. As industries demand increasingly complex and miniaturized components, this process will remain a cornerstone of advanced manufacturing, particularly for zinc alloy micro-hole applications.